Ethics and governance
Metro North Hospital and Health Service (MNHHS) is committed to the highest standards of research integrity. Research governance, including the ethical review of research, refers to the processes to ensure that research in Metro North Hospital and Health Service is conducted according to the appropriate regulatory, ethical and scientific standards. The framework for research governance is relevant to all stages of the research process.
Research approvals
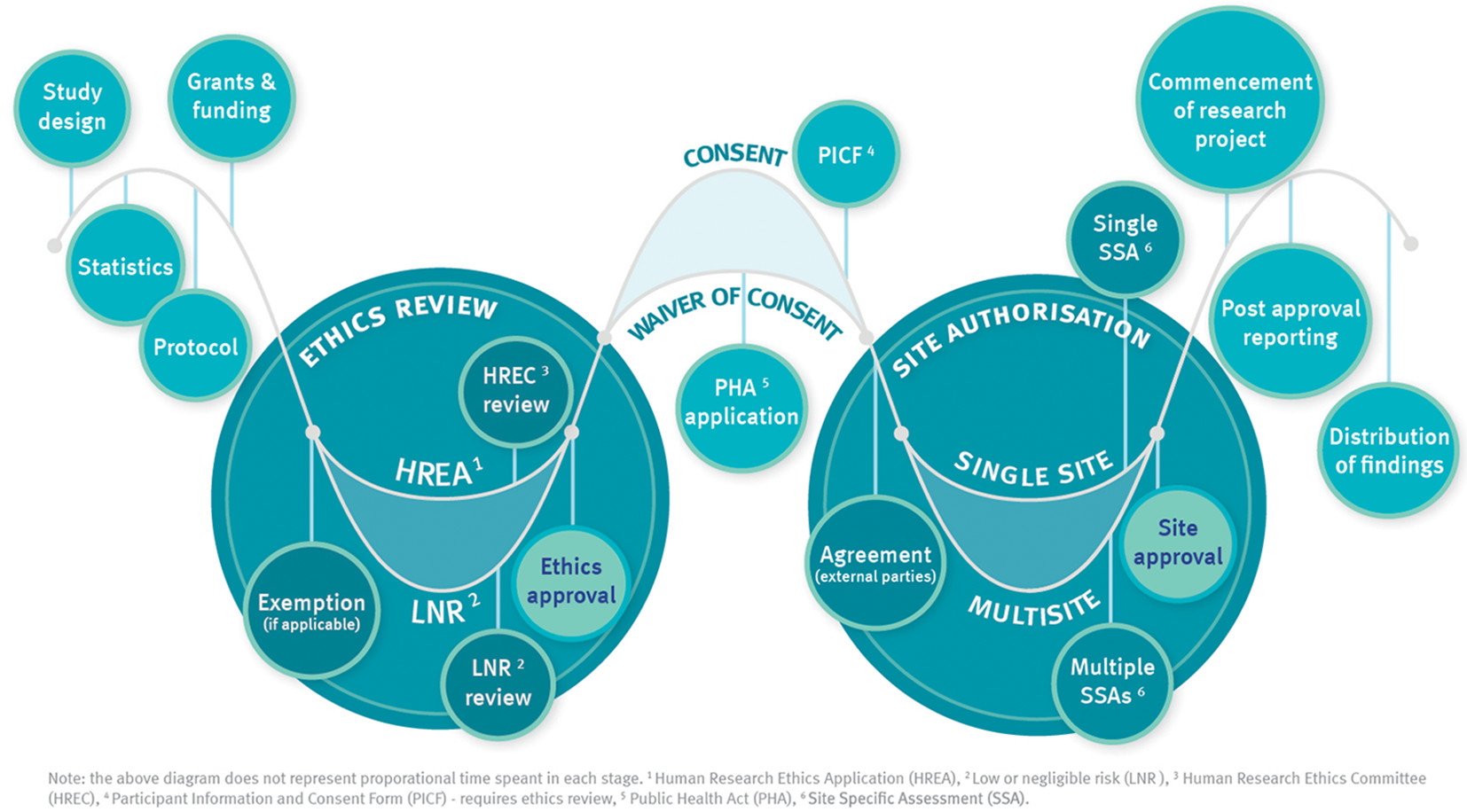
In order to conduct research in Metro North HHS, it is a requirement that all research first obtains the following:
- Ethics approval from a Human Research Ethics Committee (HREC)
- Site authorisation at each facility you are intending to do the research
The Metro North Hospital and Health Service research ethics committees and research governance officers are the contact point for obtaining relevant research approvals.
Use of confidential health information
In some cases approval to use confidential information for the purposes of research must be obtained after ethics approval and prior to site authorisation.
Post-approval reporting
While undertaking a research project, researchers have an obligation to both the participants and to the Human Research Ethics Committee (HREC) and Research Governance Officers to provide reporting and monitoring.
Metro North Research policy and procedures
The Metro North Research Policy and Procedures provide a framework to promote the responsible and ethical design, conduct and communication of research. Each is based on the principles of the National Statement on Ethical Conduct in Human Research (National Statement) and the Australian Code for the Responsible Conduct of Research (the Code), in the context of institutional policies, state and federal legislation and regulatory guidelines.
Frequently asked questions
Research process
Key steps in the research approval process are shown in the diagram below.